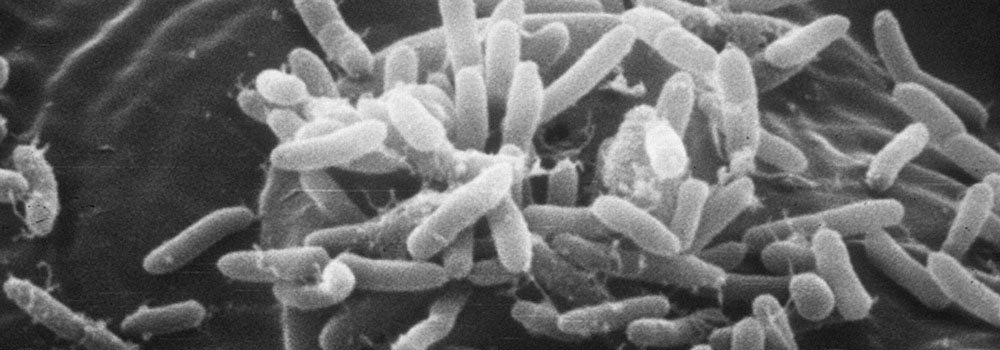
Bacteria have adapted to a huge range of environments on earth, surviving and multiplying in and on plants and animals, in rock layers deep beneath the surface, in searing desert soils, under polar ice, and under extremely high temperatures and pressures in thermal vents on the ocean floor. During the past decade, we have begun to realize that the success of these tiny, single-celled organisms may depend in large part on their ability to converse with one another using chemical signals. Cell-to-cell communication allows bacteria to coordinate their activity and thus enjoy benefits otherwise reserved for multicellular organisms.
By means of a process called quorum sensing, bacteria are able to detect when they are assembled in large numbers as opposed to when they are essentially alone. They may then adjust their behavior accordingly. Bacteria alert one another to their presence by releasing chemical molecules known as autoinducers. When a chemical of this type becomes sufficiently concentrated in the environment (for example, in an organ such as the lungs or intestinal tract), bacteria that are sensitive to it respond by turning on genes that regulate the production of certain proteins. The newly manufactured proteins, in turn, affect the behavior of the bacteria, which take advantage of one another’s presence in their efforts to survive and proliferate.
Until recently, the exchange of chemical signals was assumed to be a trait characteristic of “higher” multicellular organisms. Researchers knew of only a few cases of bacterial cell-to-cell communication and considered them the exception rather than the rule. But now scientists are realizing that this capacity is not only common but critical for bacterial survival and interaction in natural habitats.
The phenomenon of quorum sensing was first discovered in two species of bioluminescent marine bacteria,Vibrio fischeri and V. harveyi. Both of these glow-in-the-dark organisms produce light only when their quorum-sensing ability notifies them that they have reached a high cell density. They then manufacture luciferase, an enzyme concoction that facilitates a light-producing biochemical reaction. Although the two species are quite closely related, they inhabit very different niches in the ocean. V. fischeri lives in symbiotic association with a number of marine animals, producing light that host animals use for such purposes as luring prey, scaring off predators, and attracting mates. In return, V. fischeri gets to reside in the hosts’ specialized light organs, where it is provided with amino acids and other nutrients. V. harveyi, by contrast, is a free-living organism, and no one has yet figured out what advantage it derives from emitting light.
One of V. fischeri‘s most fascinating associations is with certain bobtail squids of the genus Euprymna, the best studied being the Hawaiian bobtail squid. Living in knee-deep coastal waters, this small creature buries itself in the sand during the day and comes out to hunt after dark. Its lifestyle makes the squid especially vulnerable to predation on clear, bright nights, when light shining on the animal from the moon and stars could cause it to cast a shadow and tip off predators patrolling beneath it. But through an alliance with V. fischeri, the squid has evolved a light organ that serves as a camouflaging mechanism. The amount of light emitted from this organ, located on the underside of the creature’s body, is controlled by an iris-like structure. The squid senses the intensity of light from the sky and regulates its light organ accordingly, so that the animal, seen from below, more or less matches the background.
 The squid’s light is produced by the symbiotic bacteria inhabiting the light organ. After a baby squid hatches, V. fischeri bacteria in the seawater swim through ducts leading into the immature light organ, where the hospitable conditions enable them to multiply. There the bacteria live suspended in fluid and, as part of their normal behavior, secrete an autoinducer (the chemical that signals their presence) into it. The bacteria interpret a threshold concentration of this chemical as their cue to switch on the production of light. In effect, V. fischeri bacteria alert one another that they are inside a suitable host. When dispersed in the ocean water, however, the bacteria and their autoinducer chemicals never reach critical concentrations. Then again, the bacteria probably do not gain anything by emitting light outside the squid.
The squid’s light is produced by the symbiotic bacteria inhabiting the light organ. After a baby squid hatches, V. fischeri bacteria in the seawater swim through ducts leading into the immature light organ, where the hospitable conditions enable them to multiply. There the bacteria live suspended in fluid and, as part of their normal behavior, secrete an autoinducer (the chemical that signals their presence) into it. The bacteria interpret a threshold concentration of this chemical as their cue to switch on the production of light. In effect, V. fischeri bacteria alert one another that they are inside a suitable host. When dispersed in the ocean water, however, the bacteria and their autoinducer chemicals never reach critical concentrations. Then again, the bacteria probably do not gain anything by emitting light outside the squid.
A remarkable part of this exquisite symbiosis is the way the squid keeps the bacterial culture fresh within its light organ. At sunrise, when the squid prepares to bury itself in the sand for a day of sleep, so many bacterial cells are living in its light organ that the animal cannot supply them all with adequate nutrients. The squid circumvents this problem by pumping out about 95 percent of the V. fischeri. This also reduces the level of autoinducer in the light organ below the critical threshold and causes the bacteria remaining within to stop producing light. The pumping is tuned to the squid’s circadian rhythm and is activated only at sunrise. As the day goes by, the bacteria begin to divide, their numbers increase, and more autoinducer accumulates. By nightfall, the light organ is “on” again, ready to do its job.
Quorum sensing is not restricted to glow-in-the-dark marine bacteria. In the past decade, scientists have found it in many other species, with variations in the autoinducer molecules secreted, the means by which they are detected, the biochemical reactions they trigger, and the behavior they regulate. For example, quorum sensing controls the production of virulence factors (toxins and other disease-causing agents) in numerous human and plant pathogens that have a clinical or agricultural impact. Invading bacteria may improve their odds of overcoming a host’s defenses by releasing their virulence factors simultaneously and only when they are present in great numbers. A premature release might tip off the host’s immune system.
In natural environments, bacterial species compete with one another for nutrients, for entry into hosts, and for survival under hostile conditions. Many bacterial species produce antibiotics–chemical compounds to which they themselves are immune but that kill their competitors or impede their growth. Quorum sensing enables the bacteria to coordinate the release of these antibiotics in high doses.
Quorum sensing also enhances the ability of some bacteria to acquire DNA fragments that, because of the death of some of their fellows, are up for grabs in the environment. These DNA fragments are a useful resource for repairing mutated or damaged chromosomes. Only where there is a concentrated population of bacteria is there likely to be any substantial amount of free DNA available. In this case, quorum sensing turns on the machinery that enables cells to take in this DNA.
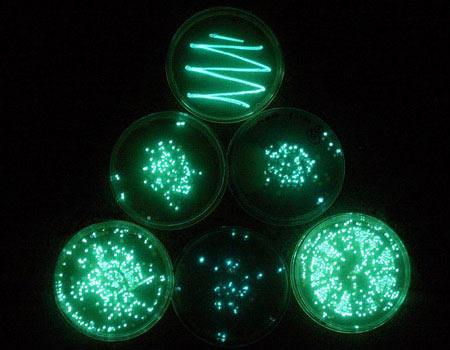 Bacterial mating, which creates a more diverse array of individuals and can spread advantageous genes through a species, seems to employ quorum sensing as well. The process involves donor cells and recipient cells. We know that in Agrobacterium tumefaciens, a species that causes tumors in susceptible plants, the donors communicate with one another through quorum sensing, but exactly what function this serves is not yet understood.
Bacterial mating, which creates a more diverse array of individuals and can spread advantageous genes through a species, seems to employ quorum sensing as well. The process involves donor cells and recipient cells. We know that in Agrobacterium tumefaciens, a species that causes tumors in susceptible plants, the donors communicate with one another through quorum sensing, but exactly what function this serves is not yet understood.
Often bacteria live in biofilms, or communities attached to a surface such as a rock in a pond or the lining of an intestine. A biofilm is surrounded by a polymer coating, or shield, that keeps the bacteria from drying out and that also resists antibiotics and other environmental assaults. The bacterial community is typically made up of several different species; as in a human metropolis, each member of the community—usually each species—has a specific job. One member, for example, may be responsible for producing the enzymes and molecular building blocks needed to create the polymer shield. Within the biofilm is a network of channels that allow water and nutrients to reach the resident bacteria and permit waste products to flow out. At least in some cases, proper formation of these channels has been shown to be dependent on quorum sensing, although the details of how this is controlled remain to be worked out.
Many bacteria are known to produce and detect several different autoinducers. For example, recent studies show that the free-living luminous bacterium V. harveyi, in addition to having the quorum-sensing system that enables it to “turn on” its glow, has a separate system that involves another autoinducer. This second chemical signal has been found in a variety of other bacteria as well. These and other findings have led to speculation that this widespread molecule is the basis of a common “language,” a bacterial Esperanto providing communication between species.
The capacity to distinguish signals both from its own kind and—through a more universal code—from others could provide a population of a particular bacterial species with valuable information. It could learn not only the cell density of its own population but also whether or not it was sharing its habitat with other species and even whether its own kind was in a majority or a minority at any given time. By adjusting its behavior, this population could then make the most of the prevailing conditions.
Many bacteria that infect humans have now been shown to produce the interspecies signal molecule. They include Escherichia coli (food poisoning), Salmonella typhimurium (food poisoning), S. typhi (typhoid fever),Haemophilus influenzae (pneumonia, meningitis, sepsis), Helicobacter pylori (peptic ulcers, stomach cancer), Borrelia burgdorferi (Lyme disease), Neisseria meningitidis (meningitis), Yersinia pestis (bubonic plague), Campylobacter jejuni (food poisoning), Vibrio cholerae (cholera), Mycobacterium tuberculosis(tuberculosis), Enterococcus faecalis (endocarditis, urinary tract infections), Streptococcus pneumoniae(pneumonia, ear inflammations), and Staphylococcus aureus (pneumonia, endocarditis, septicemia, toxic shock syndrome, meningitis, food poisoning). While for the most part the specific function served by the autoinducer in these bacteria is not yet known, there is mounting evidence that, at least in some cases, it increases virulence.
The explosion of research in quorum sensing, especially in pathogenic bacteria, is pointing the way to new biotechnological applications. If therapies could be developed that manipulate or disrupt quorum sensing, such drugs would constitute a new class of antibiotics. A new broad-spectrum antibiotic might result, for instance, if a way can be devised to undermine the interspecies signaling system.
Some novel research is focused on designing molecules that are structurally similar to autoinducers. The idea is to make a molecule that binds to the autoinducer detector of a particular bacterial species, blocking its ability to sense the appropriate signal molecule. This would prevent pathogenic bacteria from recognizing when they are assembled in great numbers and would thus avert the process that is normally triggered. Another approach is to design drugs that specifically interfere with the enzymes involved in synthesizing autoinducers, thus preventing the bacteria from sending out their signal molecules.
In some cases, host organisms already seem capable of manipulating quorum-sensing systems to their own advantage. For example, Pseudomonas aeruginosa, a bacterium present in soils and wetland habitats, poses a threat of infection to people already debilitated by cystic fibrosis, burns, cancer, or other conditions. By detecting and responding to autoinducer signals, the victim’s body may be able to hinder the secretion of this bacterium’s toxins. In other cases, hosts appear to produce molecules that mimic, and in some way interfere with, the quorum-sensing signals. This has been observed in some plants and algae.
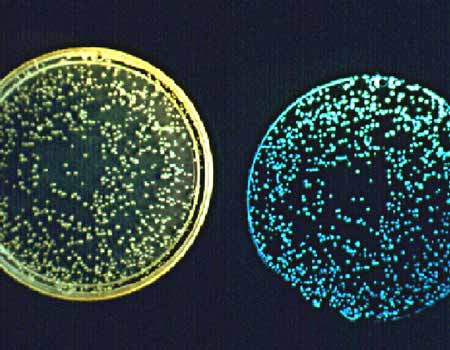 While much applied research is directed toward finding ways to disrupt quorum sensing, the process can also be exploited in a positive way. For example, cell-to-cell communication may enhance the production of antibiotics. By finding ways to promote quorum sensing, scientists may discover how to improve the commercial production of natural antibiotics, enzymes, and other biochemicals useful in the prevention and treatment of disease, for the protection of food sources, and in industrial processes.
While much applied research is directed toward finding ways to disrupt quorum sensing, the process can also be exploited in a positive way. For example, cell-to-cell communication may enhance the production of antibiotics. By finding ways to promote quorum sensing, scientists may discover how to improve the commercial production of natural antibiotics, enzymes, and other biochemicals useful in the prevention and treatment of disease, for the protection of food sources, and in industrial processes.
Whatever the practical applications, the investigation of quorum sensing promises to provide biologists with insights into a key step in the evolution of multicellular organisms. An appreciation of the molecular mechanisms that govern this bacterial process will lay the foundation for a better understanding of the development of organs and of cell-to-cell interactions and information processing in higher organisms.